2025
135. Topochemical Alkyne Nitrile Oxide Cycloaddition for Polymer Synthesis
Dr. R. Singh, A. Siddhaarthan and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202520947

134. Single-Crystal-to-Single-Crystal Synthesis of an Adaptive Two-Dimensional Polymer with Dynamic Pores
H. Balan, S. Shahanas and Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.5c13942

133. Topochemical Polymerization of Retro-Isomeric Peptides for Tuning the Polymer Structure and Properties
A. Lal, R. Rai, M.C. Madhusudhanan and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202503624

132. Isomer-Dependent Reactivity in the Solid State: Topochemical [4+4] vs. [4+2] Cycloaddition Reactions
A. Lal and Prof. Dr. K. M. Sureshan*
Chem. Sci., https://doi.org/10.1039/D5SC02327K
131. Absolute Asymmetric Synthesis of a Homochiral Polymer from an Achiral Monomer
D. Xavier, S. Pathak, Dr. C. R. Göb and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202510058

.png)
130. Single-Crystal-to-Single-Crystal Synthesis of a Rope-Ladder Polymer
H. Balan, G. Sadasivan, E. Paul and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202506699
129. Supramolecular Preorganization of Amine-functionalized Diacetylene Monomers in their Crystals Allows their Topochemical Polymerization to Polydiacetylenes Capable of CO2 Capture
A. Lal, Dr. C. Raju and Prof. Dr. K. M. Sureshan*
Chem. Eur. J., https://doi.org/10.1002/chem.202403935


128. Single-Crystal-to-Single-Crystal Synthesis of a Polymer in Two Distinct Topologies
Dr. J. R. Pathan, H. Balan, K. Das, Prof. Dr. C. M. Reddy and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202500646

127. A Malleable Collagen-Mimic that Undergoes Moisture-Induced Hardening for Gluing Hydrophilic Surfaces
Dr. R. Rai, D. Xavier, S. Pathak, Dr. F. B. Fernandez, Dr. M. Komath and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202422593

2024
126. Light-Induced Transformation of a Supramolecular Gel to a Stronger Covalent Polymeric Gel
S. K. Saleem, T. Pramod, P. Kuruva, S. V. Haridas, A. Shanmugam, Dr. Madhu Thalakulam and Prof. Dr. K. M. Sureshan*
ChemPhysChem, https://doi.org/10.1002/cphc.202400861

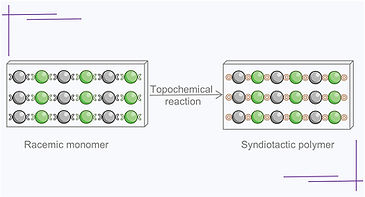
125. A Syndiotactic Polymer via Spontaneous Exoselective Single-Crystal-To-Single-Crystal Topochemical Diels–Alder Cycloaddition Reaction
S. Pathak and Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.4c11426

123. A Self-Healing Crystal That Repairs Multiple Cracks
J. R. Pathan, H. Balan, P. Commins, A. Ravi, M. B. Al-Handawi, I. C. Hou, Prof. Dr. P. Naumov* and Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.4c09334
124. Unclicking the Click: A Depolymerizable Clicked Polymer via Two Consecutive Single-Crystal-to-Single-Crystal Reactions
B. Sebastian and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202417905

121. Hierarchical single-crystal-to-single-crystal transformations of a monomer to a 1D-polymer and then to a 2D-polymer
H. Balan, Prof. Dr. K. M. Sureshan*
Nat. Commun., https://doi.org/10.1038/s41467-024-51051-z
122. Simultaneous and in situ syntheses of an enantiomeric pair of homochiral polymers as their perfect stereocomplex in a crystal
R. Khazeber, S. Pathak, Prof. Dr. K. M. Sureshan*
Nat. Commun., https://doi.org/10.1038/s41467-024-50948-z

120. Large Molecular Rotation in Crystal Changes the Course of aTopochemical Diels-Alder Reaction from a PredictedPolymerization to an Unexpected Intramolecular Cyclization
A. Lal, M. C. Madhusudhanan, Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202411165

119. Topochemistry for Difficult Peptide–Polymer Synthesis: Single-Crystal-to-Single-Crystal Synthesis of an Isoleucine-Based Polymer, a Hydrophobic Coating Material
T. Pramod, R. Khazeber, V. Athiyarath, Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.3c10779



118. Massive Molecular Motion in Crystal Leads to an Unexpected Helical Covalent Polymer in a Solid-state Polymerization
R. Khazeber, G. S. Kana, Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202316513

2023
117. Single-Crystal-to-Single-Crystal Topochemical Synthesis of a Collagen-inspired Covalent Helical Polymer
Dr. R. Rai, R. Khazeber, Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202315742

116. Two Structurally Different Polymers from a Single Monomer
J.R. Pathan, Dr. S. Bhandary, Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.3c07767
115. Topochemical Syntheses of Polyarylopeptides Involving Large Molecular Motions: Frustrated Monomer Packing Leads to the Formation of Polymer Blends
Dr. C. Raju, K. Mridula, N. Srinivasan, Dr. S. Kunnikuruvan, Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202306504


2025
137. A Hydroxyl Group Dictates Handedness, Pitch and Mechanics in a Crystalline Covalent Helical Polymer
Dr. R. Khazeber, D. Xavier and Prof. Dr. K. M. Sureshan*
Sci. Adv., https://www.science.org/doi/10.1126/sciadv.aeb5332

135. Topochemical Alkyne Nitrile Oxide Cycloaddition for Polymer Synthesis
Dr. R. Singh, A. Siddhaarthan and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202520947

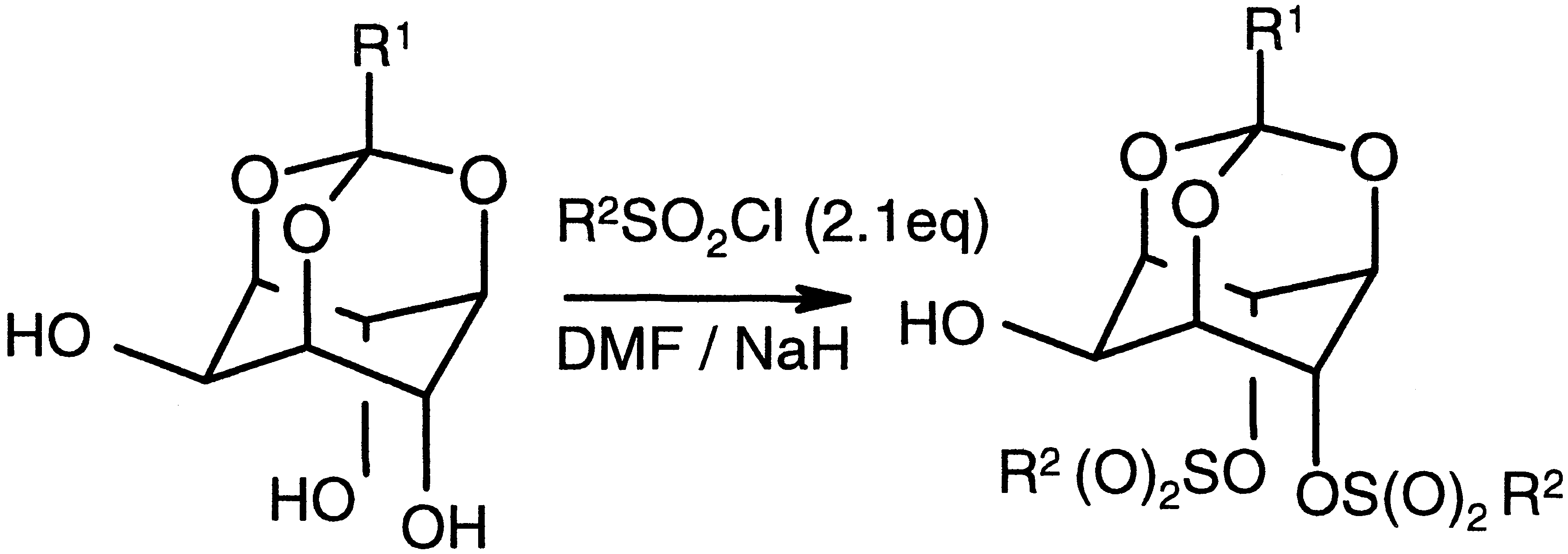
136. Topochemical Synthesis of a Syndiotactic Polymer from a Racemic Monomer
S. K. Gupta, Dr. R. Khazeber, K.S. Siddharth, A. Balakrishnan and Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.5c18292
134. Single-Crystal-to-Single-Crystal Synthesis of an Adaptive Two-Dimensional Polymer with Dynamic Pores
H. Balan, S. Shahanas and Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.5c13942

133. Topochemical Polymerization of Retro-Isomeric Peptides for Tuning the Polymer Structure and Properties
A. Lal, R. Rai, M.C. Madhusudhanan and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202503624

132. Isomer-Dependent Reactivity in the Solid State: Topochemical [4+4] vs. [4+2] Cycloaddition Reactions
A. Lal and Prof. Dr. K. M. Sureshan*
Chem. Sci., https://doi.org/10.1039/D5SC02327K
131. Absolute Asymmetric Synthesis of a Homochiral Polymer from an Achiral Monomer
D. Xavier, S. Pathak, Dr. C. R. Göb and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202510058

.png)
130. Single-Crystal-to-Single-Crystal Synthesis of a Rope-Ladder Polymer
H. Balan, G. Sadasivan, E. Paul and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202506699
129. Supramolecular Preorganization of Amine-functionalized Diacetylene Monomers in their Crystals Allows their Topochemical Polymerization to Polydiacetylenes Capable of CO2 Capture
A. Lal, Dr. C. Raju and Prof. Dr. K. M. Sureshan*
Chem. Eur. J., https://doi.org/10.1002/chem.202403935


128. Single-Crystal-to-Single-Crystal Synthesis of a Polymer in Two Distinct Topologies
Dr. J. R. Pathan, H. Balan, K. Das, Prof. Dr. C. M. Reddy and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202500646

127. A Malleable Collagen-Mimic that Undergoes Moisture-Induced Hardening for Gluing Hydrophilic Surfaces
Dr. R. Rai, D. Xavier, S. Pathak, Dr. F. B. Fernandez, Dr. M. Komath and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202422593

2024
126. Light-Induced Transformation of a Supramolecular Gel to a Stronger Covalent Polymeric Gel
S. K. Saleem, T. Pramod, P. Kuruva, S. V. Haridas, A. Shanmugam, Dr. Madhu Thalakulam and Prof. Dr. K. M. Sureshan*
ChemPhysChem, https://doi.org/10.1002/cphc.202400861

125. A Syndiotactic Polymer via Spontaneous Exoselective Single-Crystal-To-Single-Crystal Topochemical Diels–Alder Cycloaddition Reaction
S. Pathak and Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.4c11426

123. A Self-Healing Crystal That Repairs Multiple Cracks
J. R. Pathan, H. Balan, P. Commins, A. Ravi, M. B. Al-Handawi, I. C. Hou, Prof. Dr. P. Naumov* and Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.4c09334
124. Unclicking the Click: A Depolymerizable Clicked Polymer via Two Consecutive Single-Crystal-to-Single-Crystal Reactions
B. Sebastian and Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202417905

121. Hierarchical single-crystal-to-single-crystal transformations of a monomer to a 1D-polymer and then to a 2D-polymer
H. Balan, Prof. Dr. K. M. Sureshan*
Nat. Commun., https://doi.org/10.1038/s41467-024-51051-z
122. Simultaneous and in situ syntheses of an enantiomeric pair of homochiral polymers as their perfect stereocomplex in a crystal
R. Khazeber, S. Pathak, Prof. Dr. K. M. Sureshan*
Nat. Commun., https://doi.org/10.1038/s41467-024-50948-z

120. Large Molecular Rotation in Crystal Changes the Course of aTopochemical Diels-Alder Reaction from a PredictedPolymerization to an Unexpected Intramolecular Cyclization
A. Lal, M. C. Madhusudhanan, Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202411165

119. Topochemistry for Difficult Peptide–Polymer Synthesis: Single-Crystal-to-Single-Crystal Synthesis of an Isoleucine-Based Polymer, a Hydrophobic Coating Material
T. Pramod, R. Khazeber, V. Athiyarath, Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.3c10779



118. Massive Molecular Motion in Crystal Leads to an Unexpected Helical Covalent Polymer in a Solid-state Polymerization
R. Khazeber, G. S. Kana, Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202316513

2023
117. Single-Crystal-to-Single-Crystal Topochemical Synthesis of a Collagen-inspired Covalent Helical Polymer
Dr. R. Rai, R. Khazeber, Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202315742

116. Two Structurally Different Polymers from a Single Monomer
J.R. Pathan, Dr. S. Bhandary, Prof. Dr. K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.3c07767
115. Topochemical Syntheses of Polyarylopeptides Involving Large Molecular Motions: Frustrated Monomer Packing Leads to the Formation of Polymer Blends
Dr. C. Raju, K. Mridula, N. Srinivasan, Dr. S. Kunnikuruvan, Prof. Dr. K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202306504


114. Adamantoid Scaffolds for Multiple Cargo Loading and Cellular Delivery as β-Cyclodextrin Inclusion Complexes
A. Ravi, A. Pathigoolla, H. Balan, R. Gupta, G. Raj, R. Varghese, K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202307324

113. Cascading Effect of Large Molecular Motion in Crystals: A Topotactic Polymorphic Transition Paves the Way to Topochemical Polymerization
C. Raju, G. R. Ramteke, K. V. J. Jose, K. M. Sureshan*
J. Am. Chem. Soc., https://doi.org/10.1021/jacs.3c00132

112. Rational design and topochemical synthesis of polymorphs of a polymer
V. Athiyarath, L. A. Mathew, Y. Zhao, R. Khazeber, U. Ramamurty and K. M. Sureshan*
Chem. Sci., https://doi.org/10.1039/D3SC00053Bb
111. Regiospecific Synthesis of a Reprocessable Galactan-Mimic via Topochemical Polymerization
A. Ravi, S. Z. Hassan, A. Pathigoolla, A. Lal, B. Varghese, K. M. Sureshan*
ACS Sustainable Chem. Eng., https://doi.org/10.1021/acssuschemeng.3c00941

2022
110. Tuning the Regioselectivity of Topochemical Polymerization through Cocrystallization of the Monomer with an Inert Isostere
K. Hema, C. Raju, S. Bhandary, K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202210733
_tif.png)
109. Topochemical Cycloaddition Reaction between an Azide and an Internal Alkyne
C. Raju, S. Kunnikuruvan, K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202210453

108. Single-crystal-to-single-crystal translation of a helical supramolecular polymer to helical covalent polymer
R. Khazeber, K. M. Sureshan*
107. A Biomaterial-Based Porous Core–Shell Sorbent for Practical and Efficient Marine Oil Spill Recovery
C. Raju, L. A. Mathew, K. M. Sureshan*
Adv. Sustainable Syst., https://doi.org/10.1002/adsu.202100521

106. Azide···alkyne interaction: A crucial attractive force for their preorganization for topochemical cycloaddition reaction
S. Bhandary,A. Pathigoolla,M. C. Madhusudhanan, K. M. Sureshan*
105.Topochemical Postulates: Are They Relevant for Topochemical Reactions Occurring at Elevated Temperatures?
A. Ravi, Syed Z. Hassan ,S. Bhandary ,K. M. Sureshan*
Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202200954

104.Topochemical Synthesis of a Heterochiral Peptide Polymer in Different Polymorphic Forms from Crystals and Aerogels
Angew. Chem. Int. Ed., 2022, https://doi.org/10.1002/anie.202111623

2021
103. Secondary Structure Tuning of a Pseudoprotein Between β-Meander and α-Helical Forms in the Solid-State
V. Athiyarath, M. C. Madhusudhanan, S. Kunnikuruvan,, K. M. Sureshan*
Angew. Chem. Int. Ed., 2021, https://doi.org/10.1002/anie.202113129

102. Azide···Oxygen Interaction: A Crystal Engineering Tool for Conformational Locking

101. Topochemical ene-azide cycloaddition reaction
R. Khazeber, K. M. Sureshan*

100. Single-crystal-to-single-crystal synthesis of a pseudostarch via topochemical azide–alkyne cycloaddition polymerization
A. Ravi, A. Shijad, K. M. Sureshan*

99. Solvent-free and catalyst-free synthesis of cross-linkable polyfumaramides via topochemical
azide-alkyne cycloaddition polymerization
J. R. Pathan, K. M. Sureshan*

98. Novel Substrates for Kinases involved in the biosynthesis of inositol pyrophosphates and their enhancement of ATPase activity of a kinase
R. Mohanrao, R. Manorama, S. Ganguli, M. C. Madhusudhanan, R. Bhandari, K. M. Sureshan*

97. Synthesis of novel seven-membered carbasugars and evaluation of their
glycosidase inhibition potentials.
V. Athiyarath, N. J. Roy, A. T. V. Vijil, K. M. Sureshan*

96. Polymers with advanced structural and supramolecular features through
topochemical polymerization.
K. Hema, A. Ravi, C. Raju, K. M. Sureshan*

95. Topochemical polymerizations for the solid-state synthesis of organic polymers.
K. Hema, A. Ravi, C. Raju, J. R. Pathan, R. Rai, K. M. Sureshan*

93. Quantification of non‐covalent interactions in azide‐pnictogen, ‐chalcogen, and ‐halogen contacts.
M. Bursch, L. Kunze, A. M. Vibhute, A. Hansen, K. M. Sureshan, P. G. Jones, S. Grimme, D. B Werz*


2020
94. Scalable topochemical synthesis of a pseudoprotein in aerogel for water-capturing applications.
R. Mohanrao, K. Hema, K. M. Sureshan*
ACS Appl. Polym. Mater., 2020, 2, 11, 4985-4992.
92. How far are we in combating marine oil spills using phase selective organogelators?
A. M. Vibhute, K. M. Sureshan*
ChemSusChem., 2020, 13, 5343-5360

91. Designed synthesis of a 1D polymer in twist‐stacked topology via single‐crystal‐to‐single‐crystal polymerization.
V. Athiyarath, K. M. Sureshan*
Angew. Chem. Int. Ed., 2020, 59, 15580-15585

90. β‐sheet to helical‐sheet evolution induced by topochemical polymerization: Cross‐α‐amyloid‐like packing in a pseudoprotein with Gly‐Phe‐Gly repeats.
K. Hema, K. M. Sureshan*
Angew. Chem. Int. Ed., 2020, 59, 8854-8859

89. Topochemical synthesis of different polymorphs of polymers as a paradigm
for tuning properties of polymers.
R. Mohanrao, K. Hema, K. M. Sureshan*
Nat. Commun., Article number: 865 (2020)

88. Crystal‐to‐crystal synthesis of helically ordered polymers of trehalose via topochemical polymerization.
K. Hema, R. G. Gonnade, K. M. Sureshan*
Angew. Chem. Int. Ed., 2020, 59, 2897-2903.

2019
87. Topochemical azide–alkyne cycloaddition reaction.
K. Hema, K. M. Sureshan*

86. Halobenzyl alcohols as structurally simple organogelators.
A. Prathap, A. Ravi, J. R. Pathan, K. M. Sureshan*

85. Sugar-based organogelators for various applications (Invited perspective).
A. Prathap, K. M. Sureshan*
Langmuir, 2019, 35, 6005−6014.

84. Solid‐state synthesis of two different polymers in a single crystal: A miscible polymerblend from topochemical reaction.
K. Hema, K. M. Sureshan*
Angew. Chem. Int. Ed., 2019, 58, 2754-2759.

83. Spontaneous single-crystal-to-single-crystal evolution of two cross-laminated polymers.
V. Athiyarath, K. M. Sureshan*
Angew. Chem. Int. Ed., 2019, 58, 612-617.

2018
82. Synthesis and reversible hydration of a pseudoprotein, a fully organic polymeric desiccant by multiple single‐crystal‐to‐single‐crystal transformations.
R. Mohanrao, K. M. Sureshan*
Angew. Chem. Int. Ed., 2018, 57, 12435-12439.

81. Tunable mechanical response from a crystal undergoing topochemical dimerization: Instant explosionatfaster rate and chemical storage of ‘harvestable explosion’ at slower rate.
A. Ravi, K. M. Sureshan*
Angew. Chem. Int. Ed., 2018, 57, 9362-9366.

80. Organogel-derived covalent-noncovalent hybrid polymers as alkali metal ion scavengers for partial deionization of water.
A. Prathap, C. Raju, K. M. Sureshan*
ACS Appl. Mater. Interfaces, 2018, 10, 15183-15188.

79. Model molecules to classify CH∙∙∙O hydrogen-bonds.
A. M. Vibhute, U. D. Priyakumar, A. Ravi, K. M. Sureshan*
Chem. Commun., 2018, 54, 4629-4632.

78. Chirality-controlled spontaneous twisting of crystals due to thermal topochemical reaction.
R. Rai, B. P. Krishnan, K. M. Sureshan*
Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 2896-2901.

77. Three-way competition in a topochemical reaction: Permutative azide-alkyne cycloaddition reactions leading to a vast library of products in the crystal.
K. Hema, K. M. Sureshan*
CrystEngComm., 2018, 20, 1478-1482.

76. A library of multi-purpose supramolecular supergelators: fabrication of structured silica, porous plastics, and fluorescent gel.
B. P. Krishnan, K. M. Sureshan*
Chem. Asian J. 2018, 13, 187-193.

2017
75. Organogelator-cellulose composite for practical and eco-friendly marine oil spill recovery.
A. Prathap, K. M. Sureshan*
Angew. Chem. Int. Ed., 2017, 56, 9405 – 9409.

74. SN2 reactions for rapid syntheses of azido-inositols by one-pot sequence-specific nucleophilysis.
A. Ravi, S. Z. Hassan, A. N. Vanikrishna, K. M. Sureshan*
Chem. Commun., 2017, 53, 3971-3973.

73. Topochemical azide-alkyne cycloaddition reaction in gels: Size-tunable synthesis of triazole-linked polypeptides.
B. P. Krishnan, K. M. Sureshan*
J. Am. Chem. Soc., 2017, 139, 1584−1589.

2016
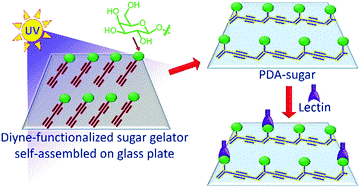
72. Organogel-assisted topochemical synthesis of multivalent glyco-polymer for high-affinity lectin binding.
B. P. Krishnan, S. Raghu, S. Mukherjee, K. M. Sureshan*
Chem. Commun., 2016, 52, 14089-14092.(Highlighted as cover article).
71. Carbasugar synthesis via vinylogous ketal: Total syntheses of (+)-MK7607, (-)-MK7607, (-)-Gabosine A, (-)-Epoxydine B, (-)-Epoxydine C, epi-(+)-Gabosine E and epi-(+)-MK7607.
S. Mondal, K. M. Sureshan*
J. Org. Chem., 2016, 81, 11635−11645.

70. Crystal-to-crystal synthesis of triazole-linked pseudo-proteins via topochemical azide–alkyne cycloaddition reaction.
B. P. Krishnan, R. Rai, A. A shokan, K. M. Sureshan*
J. Am. Chem. Soc., 2016, 138, 14824−14827.

69. Synthesis of dimeric analogs of Adenophostin A that potently evoke Ca2+ release through IP3 receptors.
A. M. Vibhute, P. Pushpanandan, M. Varghese, V. Koniecnzy, C. W. Taylor, K. M. Sureshan*
RSC Adv., 2016, 6, 86346-86351.

68. A molecular level study of metamorphosis and strengthening of gel via spontaneous polymorphic transition.
B. P. Krishnan, K. M. Sureshan*
ChemPhysChem., 2016, 17, 3062 –3067.

67. A versatile glycosylation strategy via Au(III) catalyzed activation of thioglycoside donors.
A. M. Vibhute, A. Dhaka, V. Athiyarath, K. M. Sureshan*
Chem. Sci., 2016, 7, 4259-4263.

66. A sugar-based gelator for marine oil-spill recovery.
A. M. Vibhute, V. Muvvala, K. M. Sureshan*
Angew. Chem. Int. Ed., 2016. 55, 7782-7785.

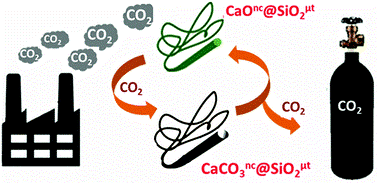
65. CaO nanocrystals grown over SiO2 microtubes for efficient CO2 capture: Organogel sets the platform.
A. Prathap, M. M. Shaijumon, K. M. Sureshan*
Chem. Commun., 2016, 52, 1342-1345. (Highlighted as cover article)
64. Topochemical synthesis of triazole-linked homobasic DNA.
A. Pathigoolla, K. M. Sureshan*
Chem. Commun., 2016, 52, 886-888. (Highlighted as cover article)

63. Semi-conducting fabrics via in situ topochemical synthesis of polydiacetylene: A new dimension to the use of organogels.
B. P. Krishnan, S. Mukherjee, P. M. Aneesh, M. A. G. Namboothiry, K. M. Sureshan*
Angew. Chem. Int. Ed., 2016, 55, 2345–2349.

2015
62. Stoichiometric sensing to Opt between gelation and crystallization.
A. Vidyasagar, K. M. Sureshan*
Angew. Chem. Int. Ed., 2015, 54, 12078-12082.

61. Triazolophostins: A library of novel and potent agonists of IP3 receptors.
A. M. Vibhute, K. M. Sureshan*
Org. Biomol. Chem., 2015, 13, 6698-6710. (Highlighted as cover article)
60. Total syntheses of five Uvacalols: Structural validation of Uvacalol A, Uvacalol B and Uvacalol C and disproval of the structures of Uvacalol E and Uvacalol G .
A. Vidyasagar, K. M. Sureshan*
Org. Biomol. Chem., 2015, 13, 3900. (Highlighted as cover article)

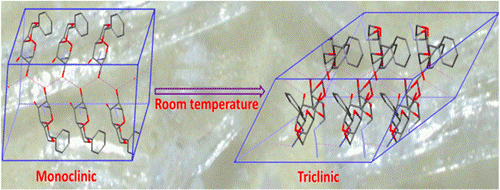
59. A spontaneous single-crystal-to-single-crystal polymorphic transition involving major packing changes.
B. P. Krishnan, K. M. Sureshan*
J. Am. Chem. Soc., 2015, 137, 1692-1696.
2014
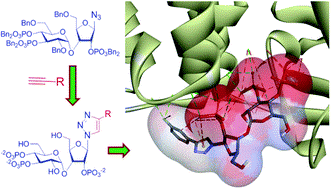
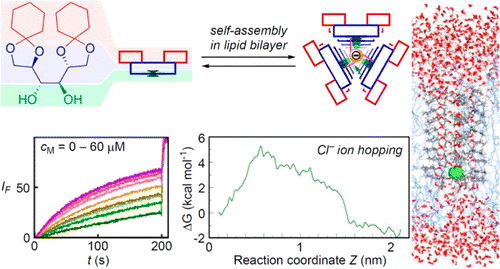
58. Hopping mediated anion transport through a mannitol-based rosette ion channel.
T. Saha, S. Dasari, D. Tewari, A. Prathap, K. M. Sureshan, A. K. Bera, A. Mukherjee, P. Talukdar
J. Am. Chem. Soc., 2014, 136, 14128–14135.
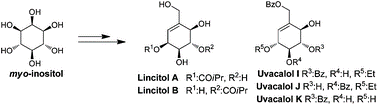
57. Total syntheses and structural Validation of Lincitol A, Lincitol B, Uvacalol I, Uvacalol J and Uvacalol K.
S. Mondal, K. M. Sureshan*
Org. Biomol. Chem., 2014, 12, 7279–7289.
56. Synthesis of triazole-linked homonucleoside polymers through topochemical azide–alkyne cycloaddition.
A. Pathigoolla, K. M. Sureshan*
Angew. Chem. Int. Ed. 2014, 53, 9522 –9525.

55. Strength from weakness: Conformational divergence between solid and solution states of substituted cyclitols facilitated by CH···O hydrogen bonding.
A. M. Vibhute, K. M. Sureshan*
J. Org. Chem., 2014, 79, 4892−4908.

54. Bio-inspired synthesis of rare and unnatural carbohydrates and cyclitols through strain driven epimerization.
R. Mohnarao, A. Asokan, K. M. Sureshan*
Chem. Commun. 50, 2014, 50, 6707--6710.

53. Reverse-CD mimics with flexible linkages offer adaptable cavity sizes for guest encapsulation.
A. Pathigoolla, K. M. Sureshan*
Chem. Commun., 2014, 50, 317-319.

52. Total synthesis and glycosidase inhibition studies of (–)-Gabosine J and its derivatives.
A. Vidyasagar, K. M. Sureshan*
Eur. J. Org. Chem., 2014, 2349–2356.

2013
51. Vinylogy in orthoester hydrolysis: Total syntheses of Cyclophellitol, Valienamine, Gabosine K, Valienone, Gabosine G, 1-epi-streptol, Streptol, and Uvamalol A.
S. Mondal, A. Prathap, K. M. Sureshan*
J. Org. Chem., 2013, 78, 7690–7700.

50. Chemoselective alcoholysis/acetolysis of trans-ketals over cis-ketals and its application in the total synthesis of the cellular second messenger, D-myo-inositol 1,4,5-trisphosphate.
A. Vidyasagar, A. Pathigoolla, K. M. Sureshan*
Org. Biomol. Chem. 2013, 11, 5443-5453.

49. A crystal-to-crystal synthesis of triazolyl linked polysaccharide.
A. Pathigoolla, K. M. Sureshan*
Angew. Chem. Int. Ed., 2013, 52, 8671-8675.

48. H2SO4-silica: An eco-friendly heterogeneous catalyst for the differential protection of myo-inositol hydroxyl groups.
A. M. Vibhute, K. M. Sureshan*
RSC Adv., 2013, 3, 7321-7329.

47. Weak becomes strong: Remarkable strength of CH...Pi hydrogen bond in the presence of OH...O hydrogen bonds in the crystal stabilization.
K. M. Sureshan*, R. G. Gonnade
CrystEngComm., 2013, 15, 1676-1679.
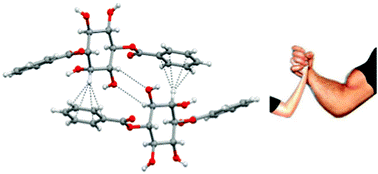
46. A versatile solvent-free azide-alkyne click reaction catalyzed by in situ generated copper nanoparticles.
A. Pathigoolla, R. P. Pola, K. M. Sureshan*
Appl. Catal. A: General, 2013, 453, 151-158.

45. Supramolecular design of a bicomponent topochemical reaction between two non-identical molecules.
B. P. Krishnan, S. Ramakrishnan, K. M. Sureshan*
Chem. Commun., 2013, 49, 1494-1496.
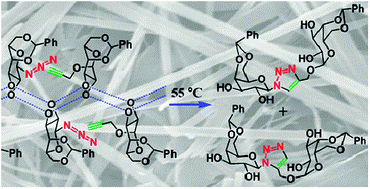
2012
44. Topochemical click reaction: Spontaneous self-stitching of a monosaccharide to linear oligomers through lattice controlled azide-alkyne cycloaddition.
P. Atchutarao, R. G. Gonnade, K. M. Sureshan*
Angew. Chem. Int. Ed., 2012, 51, 4362-4366. (Highlighted in Synfacts 2012, 8, 727).

43. Mannitol based phase selective supergelator offers a simple, viable and greener method to combat marine oil spills.
A. Prathap, K. M. Sureshan*
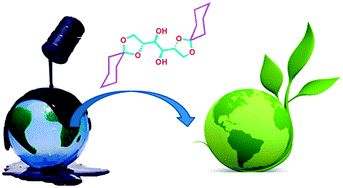
Chem. Commun., 2012, 48, 5250-5252. (Cover page article)
42. Contribution of phosphates and adenine to the potency of adenophostins at the IP3 receptor: Synthesis of all possible bisphosphates of Adenophostin A.
K. M. Sureshan, A. M. Riley, M. Thomas, S. Tovey, C. W. Taylor, B. V. L. Potter
J. Med. Chem., 2012, 55, 1706-1720.

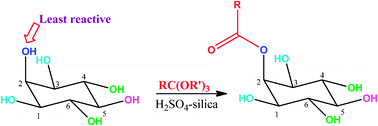
41. Regioselectivity among six secondary hydroxyl groups: Selective acylation of the least reactive hydroxyl groups of inositol.
A. M. Vibhute, A. Vidyasagar, S. Sarala, K.M. Sureshan*
Chem. Commun., 2012, 48, 2448-2450.
40. Strength from weakness: Opportunistic CH...O interactions differentially dictate the conformational fate in solid and solution states.
A. M. Vibhute, R. G, Gonnade, R.S. Swathi, K. M. Sureshan*
Chem. Commun., 2012, 48, 717-719.

39. Strength from weakness: The role of CH...N hydrogen bond in the formation of wave-like topology in crystals of aza-heterocycles.
S. Kota, A. Vidyasagar, A. Naidu, R. G. Gonnade, K. M. Sureshan*
CrystEngComm., 2012, 14, 519-524.

2011
38. Soft optical devices from self-healing gels formed by oil and sugar based organogelators.
A. Vidyasagar, K. Handore, K. M. Sureshan*
Angew. Chem. Int. Ed., 2011, 50, 8021-8024.
Highlighted as RESEARCH HIGHLIGHTS in Nature: Nature, 2011, 475, 427.
Also Highlighted in Optics & Photonics Focus 2011,15, story 2
Angewandte Highlights Angew. Chem. Int. Ed., 2012, 51, 1760.

Till 2010
37. Selective determinants of inositol 1,4,5-trisphosphate and adenophostin A interactions with type-I inositol 1,4,5-trisphosphate receptors.
A. M. Rossi, K. M. Sureshan, A. M. Riley, B. V. L. Potter, C. W. Taylor
British J. Pharmacol., 2010, 161, 1070-1085.

2009
36. Total synthesis of cyclitol based natural products from myo-inositol: brahol and pinpollitol.
K. M. Sureshan*, T. Murakami, Y. Watanabe
Tetrahedron, 2009, 65, 3998-4006.

35. Activation of IP3 receptors by synthetic bisphosphate ligands.
K. M. Sureshan, A. M. Riley, A. M. Rossi, S. C. Tovey, S. G. Dedos, C. W. Taylor, B. V. L. Potter
Chem. Commun., 2009, 1204-1206. (Highlighted as Prospected Article)

34. Regioselective O-acylation of myo-inositol 1,3,5-orthoesters: Dependence of regioselectivity on the stoichiometry of the base.
K. M. Sureshan, M. S. Shashidhar
Tetrahedron, 2009, 65, 2703-2710.

2008
33. Efficient syntheses of optically pure chiro- and allo- inositol derivatives, azidocyclitols and aminocyclitols from myo-inositol.
K. M. Sureshan*, K. Ikeda, N. Asano, Y. Watanabe
Tetrahedron, 2008, 64, 4072-4080.

32. Strength from weakness: CH…π stabilized conformational tuning of benzyl ethers and a consequent co-operative edge-to-face CH…π network.
K. M. Sureshan*, T. Uchimaru, Y. Yao, Y. Watanabe
CrystEngComm., 2008, 10, 493-496. (Highlighted as Hot Article)

31. 2-Position base modified analogs of adenophostin A as high affinity agonists of the D-myo-inositol trisphosphate receptor: In vitro evaluation and molecular modeling.
K. M. Sureshan, M. Trusselle, S. C. Tovey, C. W. Taylor, B. V. L. Potter
J. Org. Chem., 2008, 73, 1682-1692. (Highlighted as Featured Article)

2007
30. Rapid and efficient routes to phosphatidylinositols 3,4,5-trisphosphates via myo-inositol orthobenzoate.
K. M. Sureshan, A. M. Riley, B. V. L. Potter
Tetrahedron Lett., 2007, 48, 1923-1926.

2006
29. Guanophostin A: Synthesis and evaluation of a high affinity agonist of the D-myo-inositol 1,4,5-trisphosphate receptor.
K. M. Sureshan, M. Trusselle, S. C. Tovey, C. W. Taylor, B.V. L. Potter
Chem. Commun., 2006, 2015-2017. (Highlighted as the Cover Picture)

2005
28. Solid and solution state conformations of (±)-3-O-acetyl-1,2:4,5-di-O-isopropylidene-allo-inositol and (±)-3-O-acetyl-1,2:4,5-di-O-isopropylidene-6-O-methyl-allo-inositol.
K.M. Sureshan*, Y. Watanabe
Carbohydr. Res., 2005, 340, 2311-2318.

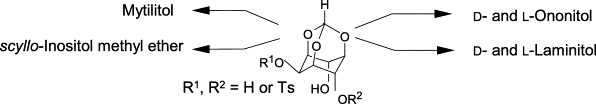
27. Sulfonate protecting groups. Synthesis of O- and C-methylated inositols: D- and L-ononitol, D- and L-laminitol, mytilitoland scyllo-inositol methyl ether.
M. P. Sarmah, M. S. Shashidhar, K. M. Sureshan, R.G. Gonnade, M. M. Bhadbhade
Tetrahedron, 2005, 61, 4437-4446.
26. Establishment of the structure of pinpollitol by total synthesis of the proposed putative structures.
K. M. Sureshan*, T. Murakami, Y. Watanabe
Synlett., 2005, 769-772.

25. Short S=O…C=O contacts associate diastereomers of 2,4(6)-di-O-benzoyl-6(4)-O-[(1S)-10-camphorsulfonyl]-myo-inositol 1,3,5-orthoformates in their inclusion complexes.
K. Manoj, K. M. Sureshan, R. G. Gonnade, M. M. Bhadbhade, M. S. Shashidhar
Cryst. Growth Des., 2005, 5, 833-836.

24. Resolution of synthetically useful myo-inositol derivatives using the chiral auxiliary O-acetylmandelic acid.
K. M. Sureshan*, Y. Kiyosawa, F. Han, S. Hyodo, Y. Uno, Y. Watanabe
Tetrahedron: Asymmetry, 2005, 16, 231-241.

2004
23. O-acetylmandelic acid as a reliable chiral anisotropy reagent for the determination of absolute configuration of alcohols.
K.M. Sureshan*, T. Miyasou, S. Miyamori, Y. Watanabe
Tetrahedron: Asymmetry, 2004, 15, 3357-3364.

22. Probing gelation at the molecular level: head-to-tail hydrogen-bonded self-assembly of an inositol-based organogelator.
K. M. Sureshan*, K. Yamaguchi, Y. Sei, Y Watanabe
Eur. J. Org. Chem., 2004, 4703-4709.

21. Efficient routes to optically active azido-, amino-, diazido- and diaminocyclitols with chiro- and allo- configuration from myo-inositol.
K. M. Sureshan*, K. Ikeda, N. Asano, Y. Watanabe
Tetrahedron Lett., 2004, 45, 8367-8370.

20. Topochemical transketalization reaction driven by hydrogen bonding.
K. M. Sureshan*, T. Murakami, T. Miyasou, Y. Watanabe
J. Am. Chem. Soc., 2004, 126, 9174-9175.

19. Solid and solution state conformation of 1L-1-O-acetyl-2,3:5,6-di-O-isopropylidene-chiro-inositol.
K. M. Sureshan*, T. Miyasou, Y. Watanabe
Carbohydr. Res., 2004, 339, 1803-1807.

18. Crystal structure of 1L-1,2:4,5-di-O-isopropylidene-allo-inositol; A comparison of its conformation in solid and solution states.
K. M. Sureshan*, T. Miyasou, Y. Watanabe
Carbohydr. Res., 2004, 339, 1551-1555.

17. Total synthesis of the proposed structure of brahol and the structural revision.
K. M. Sureshan, T. Miyasou, Y. Watanabe
Tetrahedron Lett., 2004, 45, 3197-3201.

16. An efficient route to optically active inositol derivatives via the resolution of myo-inositol 1,3,5-orthoformate: a short synthesis of D-myo-inositol-4-phosphate.
K. M. Sureshan, Y. Watanabe
Tetrahedron: Asymmetry, 2004, 15, 1193-1198.

15. Simple and efficient routes to optically active chiro and allo-inositol derivatives from myo-inositol.
K. M. Sureshan, Y. Watanabe
Synlett., 2004, 493-496.

14. Crystal structure, solid state and solution conformation of 1D-1,4-di-O-[(S)-O-acetylmandeloyl]2,3:5,6-di-O-isopropylidene-myo-inositol.
K. M. Sureshan*, T. Miyasou, Y. Watanabe
Carbohydr. Res., 2004, 339, 807-811.

13. Is O-acetylmandelic acid a reliable chiral anisotropy reagent?
K. M. Sureshan, T. Miyaso, M. Hayashi, Y. Watanabe
Tetrahedron: Asymmetry, 2004, 15, 3-7.

2003
12. A simple and practical resolution of 1,2:4,5-di-O-isopropylidene-myo-inositol.
K. M. Sureshan, T. Yamasaki, M. Hayashi, Y. Watanabe
Tetrahedron: Asymmetry, 2003, 14, 1771-1774.

11. Regioselective protection and deprotection of inositol hydroxyl groups.
K. M. Sureshan, M. S. Shashidhar, T. Praveen, T. Das
Chem. Rev., 2003, 103, 4477-4503.
10. Sulfonate protecting groups: Synthesis of D- and L-myo-inositol-1,3,4,5-tetrakisphosphate precursors by a novel Silver (I) oxide mediated O-alkylation of 2,4(6)-di-O-acyl-6(4)-O-sulfonyl-myo-inositol 1,3,5-orthoformate derivatives through intramolecular assistance of the sulfonyl group.
K. M. Sureshan, T. Das, M. S. Shashidhar, R. G. Gonnade, M. M. Bhadbhade
Eur. J. Org. Chem., 2003, 1035-1041.
2002
09. Regioselective sulfonylation of orthoesters of myo-inositol: Formal synthesis of both D- and L-myo-inositol 1,3,4,5 tetrakisphosphate.
K. M. Sureshan, M. S. Shashidhar
Trends Carbohydr. Chem., 2002, 8, 77-86.
08. Sulfonate protecting groups. Regioselective sulfonylation of myo-inositol orthoesters: Improved synthesis of precursors of D- and L-myo-inositol 1,3,4,5-tetrakisphosphate, myo-inositol-1,3,4,5,6-pentakisphosphate and related derivatives.
K. M. Sureshan, M. S. Shashidhar, T. Praveen, R. G. Gonnade, M. M. Bhadbhade
Carbohydr. Res., 2002, 337, 2399-2410
07. Cyclitol based metal complexing agents: Effect of the relative orientation of oxygen atoms in the ionophoric ring on the cation binding ability of myo-inositol based crown ethers.
K. M. Sureshan, M. S. Shashidhar, A. J. Varma
J. Org. Chem. 2002, 67, 6884-6888.

06. Silver (I) oxide – Silver halide mediated alcoholysis of O-benzoyl-myo-inositol 1,3,5-orthoformates: Intramolecular assistance by the sulfonyl group.
T. Praveen, T. Das, K. M. Sureshan, M. S. Shashidhar, U. Samanta, D. Pal, P. Chakrabarti
J. Chem. Soc. Perkin Trans 2, 2002, 358-365.

2001
5.Neutral complexing agents with a cyclitol core. Effect of the relative orientation of the sidearms and end groups on the cation binding ability of myo-inositol based podands
K. M. Sureshan, M.S. Shashidhar, A. J. Varma
J. Chem. Soc., Perkin Trans. 2, 2001, 2298–2302

04. A highly selective host-guest system formed and stabilized due to concerted halogen-oxygen and C-H ...O non-bonded interactions: X-ray structures of racemic 1,2,3,4,5-penta-O-benzoyl-6-O-tosyl-myo-inositol-dihalomethane (CH2X2, x=Cl and Br) inclusion complexes.
K. M. Sureshan, R. G. Gonnade, M. S. Shashidhar, V. G. Puranik, M. M. Bhadbhade
Chem. Commun., 2001, 881-882.
03. Sulfonate protecting groups. Regioselective O-sulfonylation of myo-inositol orthoesters.
K. M. Sureshan, M. S. Shashidhar
Tetrahedron Lett. 2001, 42, 3037-3039.